In the wake of the COVID-19 pandemic, the term “Fast Track” has become part of our everyday vernacular. Originally established by the U.S. Food and Drug Administration (FDA) to accelerate the development and review of drugs for treating serious conditions, Fast Track has recently faced significant public scrutiny. Critics argue that it has turned into a “money-printing license” for Big Pharma at the expense of thorough scientific validation. But is this viewpoint entirely fair? Let’s delve into the complexities of fast-tracking drug approvals.
The Spirit of Fast Track
When lives are at stake, time is of the essence. The traditional drug approval process, which often spans several years, is not conducive to addressing immediate health crises. Conditions like AIDS, Alzheimer’s, cancer, and indeed, emerging pandemics, demand swift action. Fast Track aims to bridge this gap by expediting the review process without compromising on safety.
The Murky Waters of Fast Decisions
That said, speed inevitably raises concerns. When regulatory bodies expedite approvals, there’s a fine balance to strike between urgency and caution. Critics argue that the accelerated timeline means there’s less time to assess long-term side effects or the true efficacy of a drug. Given that pharmaceutical companies stand to gain financially, skepticism arises over whether these fast-tracked approvals are motivated by profit rather than public health.
What About Transparency?
Another concern is transparency in the approval process. Critics want assurance that expedited decisions are made based on comprehensive data, rather than incomplete studies or computational models alone. Open discourse and public scrutiny are vital for maintaining trust, especially when new technologies like mRNA vaccines are introduced.
Follow the Money — Is Profit Driving Fast Track Approvals?
The role of financial interests in healthcare decisions is an issue that has long elicited debate, but perhaps never more so than now. While public health should always be the primary focus of any regulatory body, the admission that financial considerations have become a significant factor in fast-tracking drug approvals raises ethical questions. The argument that without Fast Track approvals, research could move to other countries, resulting in billions in lost revenue, may be valid from an economic standpoint. But does it stand up to ethical scrutiny?
Balancing Act: Public Health vs. Profit
The quest for revenue should not compromise the quest for safety and efficacy. While keeping the pharmaceutical industry competitive is an economic imperative, it shouldn’t be pursued at the expense of rigorous scientific review. The tension between public health and profit is not new, but when economic considerations begin to tip the scales, it’s time for a reassessment.
The Slippery Slope of Financial Incentives
The real danger lies in the potential for a slippery slope where financial incentives begin to take precedence over scientific rigor. If regulatory bodies are swayed by economic factors, there’s a risk that the very essence of these organizations — to protect public health — may be compromised.
International Implications
The notion that research could move to other countries if Fast Track approvals aren’t facilitated has international implications. While keeping research and revenue within one’s borders might be a national interest, it should not come at the cost of potentially lowering approval standards. After all, diseases know no borders; if safety is compromised in one country, the repercussions could be global.
Public Trust and Transparency
The core issue here is transparency. If financial considerations are part of the decision-making process, they should be openly acknowledged and debated. Financial stakes and public health are not mutually exclusive, but a transparent dialogue is essential to balance them effectively.
Conclusion
If the catalyst for fast-tracking approvals is indeed financial, this raises the question: at what cost? Ethical considerations must always be at the forefront of any public health decision. As we scrutinize the Fast Track approval process, we must ask whether it aligns with the principles that should guide healthcare — efficacy, safety, and the greater public good. While financial considerations can’t be wholly dismissed, they should never eclipse the primary mission of healthcare regulators: to protect and promote public health.
Navigating the Road Ahead
Fast-tracking isn’t inherently good or bad; it’s a tool that can be wielded wisely or recklessly. Therefore, while questioning the motivations behind fast-tracked approvals is valid, it’s also crucial to appreciate the necessity for speed in certain medical scenarios.
We must call for rigorous oversight, transparency, and stringent conflict-of-interest rules to maintain the integrity of the fast-tracking process. While skepticism is healthy, it should be informed by verified data and peer-reviewed studies rather than speculative claims.
In an increasingly complex healthcare landscape, Fast Track serves a purpose but also warrants scrutiny. It’s not a black-and-white issue but one that demands ongoing vigilance and a commitment to open, evidence-based dialogue. Our collective well-being depends on it.
By navigating the nuances of Fast Track and related regulatory measures, society can work towards a healthcare system that is both responsive and responsible. But to get there, we must base our criticisms, and our faith, on factual evidence.
The key takeaway? Fast-tracking is a tool in our healthcare arsenal, but like any tool, it must be used responsibly. Critics and proponents alike should unite in demanding a transparent, scientifically rigorous process that puts public health first.
The saying goes, “Those who cannot learn from history are doomed to repeat it.” But what happens when history itself becomes a puzzle, its pieces missing or concealed? This question looms large when considering the noticeable absence of U.S. Food and Drug Administration (FDA) historical data prior to 2019 on the Wayback Machine, a digital archive of the World Wide Web.
What Does Missing Data Mean?
This digital void sparks a multitude of questions. If such information has indeed been removed or is not publicly available, the fundamental tenets of transparency and trust are at risk. The absence of historical data becomes even more concerning in light of recent updates to the FDA’s Fast Track approval process, coinciding with the onset of the COVID-19 pandemic.
Public Perception and the Importance of History
The FDA plays a crucial role in safeguarding public health, and it operates under a lens of public scrutiny. That lens becomes clouded when we lack access to historical documents that can provide context or justify recent changes. Without historical records, the public may lose a valuable yardstick against which to measure and evaluate current policies and decisions.
When Trust Is Compromised
In any democratic society, transparency isn’t just an ideal; it’s a requirement for the functioning of institutions that hold significant power. A lack of accessible historical data from a body as influential as the FDA could lead to a breakdown of public trust. And once trust is eroded, rebuilding it is a herculean task, especially in the age of information where skepticism runs high.
The Need for Accountability
If it is true that this information has been intentionally removed or concealed, it raises serious questions about accountability. Who made this decision? What were the motivations behind it? Were there conflicts of interest involved? Such gaps make it challenging to hold an institution accountable, leaving room for speculations, some of which may veer into the realm of conspiracy theories.
Moving Forward
The situation calls for immediate clarification from the FDA. Clear, direct communication about why this data is missing and what steps are being taken to restore it—or provide valid reasons for its removal—can go a long way in calming public fears.
Final Word
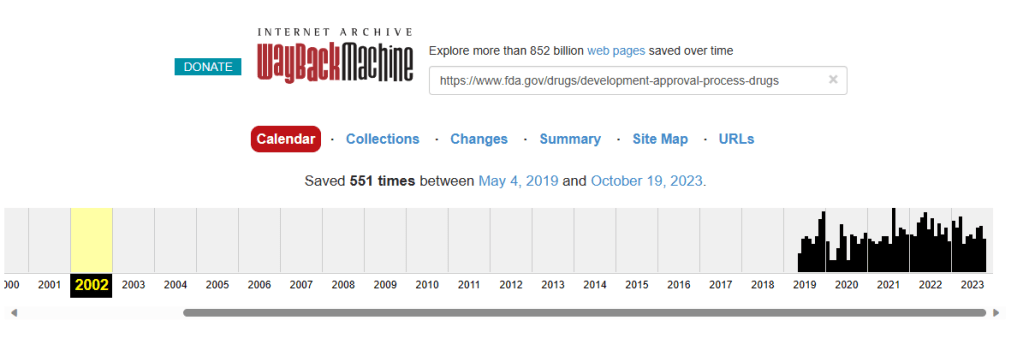
While missing data on the Wayback Machine might seem like a small issue, its implications could be far-reaching, affecting the very trust that we place in the institutions meant to protect us. As the conversation around Fast Track intensifies, this unsettling absence of historical information adds a troubling layer to an already complex debate. It is a layer that we must diligently peel back to get to the truth, whatever that may be.

Leave a comment